What are fluids?
Matter exists in several states. These states are known as physical states or states of matter. The first three states of matter are well known because we can experience them in everyday life. They are solid, liquid and gaseous (Figure 3-3). “Other” states are not considered at this time because they occur only in severe physical conditions.

Matter, whether in its liquid or gaseous state, is a fluid, and although liquids and gases react under different conditions, they have the basic properties of liquids. That is, when a shear force is applied to it, a liquid is constantly deformed. Unlike solids, which do not change much in response to a force, liquids deform and deform until a force is applied (Figure 2-3). When closed in a closed container, the liquid takes the form of a container. An enclosed liquid occupies only the bottom of the container, not all available space such as gas.

Continuous hypothesis
Liquids, like solids, are molecules that interact with each other. Tracing the velocity and position of each of these molecules is almost impossible, so a hypothesis is made to simplify models and calculations in fluid mechanics. This hypothesis is a continuous hypothesis, it is assumed that the effect of individual properties of molecules is negligible compared to the properties of the whole liquid. Thus, according to the continuous hypothesis, a low-volume element (∙ ∙) defines properties such as pressure, temperature, density, and velocity (Figure 3-4). The continuous hypothesis also requires that these properties constantly change between two adjacent volume elements. Hence, the continuous hypothesis requires that the fluid be continuous over its entire volume instead of being made up of individual molecules.
What are the main characteristics of liquids?
The four main characteristics of liquids are density, specific gravity, dynamic viscosity and vapor pressure. This section details these four features.
Density
The density of a fluid is its mass ratio per unit volume, ie
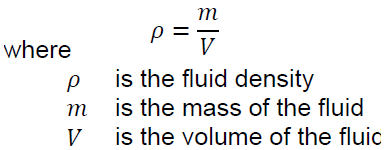
The density of a substance varies with pressure. This change is usually small for liquids and solids, but very important for gases because they are very compact. Increasing the pressure applied to the gas greatly affects its density. In contrast, liquids are relatively incompressible, and increasing the pressure does not significantly change their density. It has a density of kilograms per cubic meter (kilograms per cubic meter) in SI units and pounds per cubic foot (lb / ft3) in normal US units. The density of solids and liquids also changes with temperature. If you need to compress a sub-position at a certain temperature, refer to density as a function of the temperature table.
Special Weight
Specific gravity, or relative density, is the ratio of the density of a substance to the density of an equal volume of water:
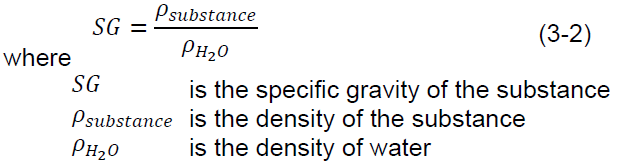
Dynamic viscosity
The viscosity of a fluid is its capacity to resist deformation. In addition, viscosity measures how easily a liquid flows. Dynamic viscosity has a secondary Pascal unit (Pa) in SI units and a pound force per second (lbf / ft ·) in normal US units. Dynamic viscosity is sometimes measured in CGS. Unit of toxins (P), where 1P is 0.1 Pa · s or 0.067 lbf / ft · s. Temperature has a significant effect on viscosity. The viscosity of liquids decreases with increasing temperature, unlike the viscosity of gases, which increases with increasing temperature.
steam pressure
Vapor pressure is the pressure created by the vapor of a liquid or solid. When the vapor is in equilibrium with the solid or liquid phase of the substance, the vapor pressure is measured for a given temperature. Steam pressure is a pressure, so it has units in kilopascals (kPa) per unit SI and pounds per square inch (psi) in normal US units.
Compressibility
Another difference between liquids and gases is their compressibility. Compressibility is the capacity of liquids to reduce volume when pressed. Liquids are relatively non-compressible while gases can be compressed. The non-compressibility of liquids indicates that their pressure increases rapidly when enclosed and pressurized. Hydraulic systems use this property of liquids to create very high pressures and transfer huge amounts of energy to other elements.
Hydrostatic pressure
Pressure is the amount of force applied per unit area:

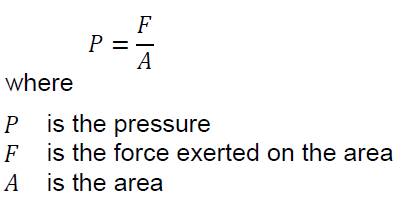
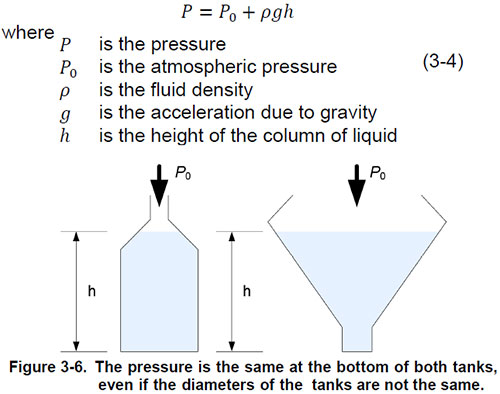
An ideal liquid cannot withstand shear force. Therefore, the forces in a small volume element are only equal to the normal forces, as Figure 3-5 shows. At rest, the pressure that a liquid exerts on a small volume element depends only on the weight of the liquid above that element. The pressure due to the weight of the liquid is proportional to the depth of the pressure measurement and only to this depth. The shape of the ship has no effect on hydrostatic pressure. For example, if two tanks are full of water but have different diameters, the hydrostatic pressure is the same at the bottom of both tanks due to the weight of the water (Figure 3-6). The absolute pressure at the bottom of the liquid column is the amount of atmospheric pressure and the hydrostatic pressure due to the weight of the liquid. Is a mathematical expression used to calculate the pressure at the bottom of a ship
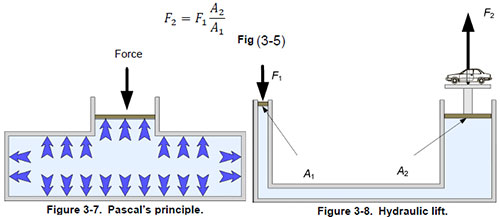
Pascal’s law
Blaise Pascal (1662-1623) The French mathematician, physicist, and philosopher deduced from the fact that a fluid column withstands a pressure that differs only in height from the fluid column, the external pressure exerted on an enclosed fluid, to any Part of the fluid fades.
Pressure measurement
Units of pressure measurement
Equipped with instrumentation training and process control systems, it puts pressure on SI units and conventional US units. The unit of pressure is SI Pascals (Pa). One Pascal (1 Pa) is a Newton (1 N) force applied to an area of one square meter (1 square meter). Therefore, 1 Pa is equal to 1 N / m2. Because most measurements are in the order of 1 × 103 Pa, most instruments perform readings in kilopascals (kPa) instead of Pascals, where one kilopascal (1 kPa) equals 1000 Pa. Pressure in normal US units. Pound force unit per square inch (PSI). One pound of force per square inch (1 PSI) is one pound of force (1 pound) applied to an area of one square inch (1 inch by 2). Some instruments also play using other units such as tape. One bar (1 bar) is equal to 100 kPa or 14.5 psi.
Head pressure
As mentioned earlier, the hydrostatic pressure at a point in a ship depends on the height of the liquid above that point. For two points on a ship, the pressure difference, ∆, is proportional to the distance, clock, between these two points (Figure 3-9). Therefore, there is a pressure difference
 PressureThe height of a liquid column to create a certain pressure must be the head or the pressure head. For a given pressure, the height is not equal to the height of water with the same height as mercury. This is because the pressure drop varies with the density of the liquid. Therefore, you must specify the type of fluid used as a reference as well as the temperature when expressing the pressure as the height of a liquid column. For high pressure, the height equivalent of a liquid column is too high to be useful for comparison. For example, 1000 kPa (145 psi) is equivalent to 102 meters of water (4015 inches of water). For this reason, the pressure head is primarily used to measure low pressure. The pressure head has units of meters (meters) or centimeters (centimeters) of water or mercury. In conventional US units, feet (feet) or inches (in) of water or mercury are common units of pressure. A pressure of 1 kPa corresponds to a head of 0.102 m of water at 4 ° C. As a result, a column 0. 102 meters of water at 4 degrees Celsius creates a pressure of 1 kPa at the bottom. Similarly, a pressure of 1 psi corresponds to a head of 27.7 in water at 39 degrees Fahrenheit. As a result, a 27.7 column in a water column at 39 degrees Fahrenheit creates a pressure of 1 psi at the end.
PressureThe height of a liquid column to create a certain pressure must be the head or the pressure head. For a given pressure, the height is not equal to the height of water with the same height as mercury. This is because the pressure drop varies with the density of the liquid. Therefore, you must specify the type of fluid used as a reference as well as the temperature when expressing the pressure as the height of a liquid column. For high pressure, the height equivalent of a liquid column is too high to be useful for comparison. For example, 1000 kPa (145 psi) is equivalent to 102 meters of water (4015 inches of water). For this reason, the pressure head is primarily used to measure low pressure. The pressure head has units of meters (meters) or centimeters (centimeters) of water or mercury. In conventional US units, feet (feet) or inches (in) of water or mercury are common units of pressure. A pressure of 1 kPa corresponds to a head of 0.102 m of water at 4 ° C. As a result, a column 0. 102 meters of water at 4 degrees Celsius creates a pressure of 1 kPa at the bottom. Similarly, a pressure of 1 psi corresponds to a head of 27.7 in water at 39 degrees Fahrenheit. As a result, a 27.7 column in a water column at 39 degrees Fahrenheit creates a pressure of 1 psi at the end.
Types of pressure measurements
The pressure is always measured relative to the reference pressure. The reference pressure can be either vacuum or atmospheric pressure. You can also measure pressure relative to other pressures.
Absolute pressure
The word vacuum is derived from the Latin word vacuus and means empty. The space vacuum is completely free of matter, and since there must be a substance to create pressure, there is no pressure in the vacuum. Therefore, the vacuum pressure is exactly 0 kPa (0 psi). The pressure measured with respect to the vacuum pressure is the absolute pressure. Absolute pressure is always positive because its reference pressure is 0 kPa (0 psi). The SI unit is the absolute pressure in Pascals, but when you refer to an absolute pressure, you must specify that it is an absolute pressure (for example: the gas in the tank has an absolute pressure of 300 kPa). In normal US units, the absolute pressure is in units of pounds per square inch, absolute, which stands for psia (for example: the gas in the tank is 43.5 psia).
Barometer
Most instruments use local atmospheric pressure as a reference instead of a vacuum as a reference for measuring pressure. At sea level, the average pressure due to the weight of the air is 101325 Pa or 101.3 kPa (14.7 psia). Locally, atmospheric pressure depends on altitude and actual atmospheric conditions. The difference between the measured pressure and the local atmospheric pressure is the barometer. The barometer reading is about reading the absolute pressure minus the local atmospheric pressure. The barometer is zero if the pressure measured is local atmospheric pressure. If the measured pressure is below the local atmospheric pressure and if it is positive, it is negative. The SI unit is the Pascal barometer. However, as in the case of absolute pressure, you must specify that you are referring to a barometer (for example: the gas in the tank is at a pressure of 200 kPa). In typical US units, a barometer has pounds per square inch, which is abbreviated to psig (for example: The gas in the tank is at a pressure of 29 psig). Figure 3-10 shows the difference between absolute pressure and barometer.
Differential pressure
Some pressure gauges measure pressure at two points, resulting in a difference in pressure. The pressure difference between two points is often referred to as the differential pressure. The pressure gauge is an example of a differential pressure gauge because it measures the difference between the measured pressure and the atmospheric pressure. The SI unit for differential pressure is Pascal. For more clarity you can specify that this is a differential pressure (for example: the differential pressure between the top and bottom of the tank is 200 kPa). In normal US units, the differential pressure in pounds per square inch is the differential, abbreviated psid (for example, the differential pressure between the top and bottom of tanks is 29 psid).
Brief definitions:
Atmospheric pressure
Atmospheric pressure is the force exerted by the Earth’s atmosphere. Atmospheric pressure at sea level is equivalent to 14,695 psia. The amount of atmospheric pressure decreases with increasing altitude.
air pressure
The barometer is the same as atmospheric pressure.
Hydrostatic pressure
Hydrostatic, pressure is observed in fluid level applications. Hydrostatic pressure is the pressure below the surface of a liquid that is exerted by the above liquid.
Line pressure
Line pressure is simply the amount of pressure or force per unit area applied by a current parallel to the pipe wall on the surface.
Static pressure
Static pressure is the same as line pressure.
Working pressure
Working pressure is also called linear or static pressure.
Absolute pressure
Absolute pressure is a unit pressure measurement referring to a complete or complete vacuum. Absolute pressure is the measurement of process pressure over a complete vacuum or 0 psia. Absolute zero pressure (0 psia) indicates a complete lack of pressure – for example, a complete vacuum.
Barometer
A barometer is a single pressure gauge that shows the high atmospheric pressure. The barometer shows a positive difference between the measured pressure and the available atmospheric pressure. You can convert the barometer to absolute pressure by adding the amount of real atmospheric pressure to the barometer reading. For example 10 psig equals 24-7 psia 0 psig equals 14.7 psia.
Vacuum
Vacuum pressure is a measure of individual pressure, which also refers to atmospheric pressure. Vacuum pressure is a measure of the amount of process pressure depression below atmospheric pressure. Vacuum pressure is generally measured in centimeters or inches of H20. For example, 14.7 psia is equivalent to 407.5 inches H20. Thus, the 10-inch pressure of the H20 vacuum indicates that the process pressure is 10 inches below atmospheric pressure. Or 10 inches of vacuum H20 is equivalent to 397.5 inches of absolute H20. Vacuum pressure is usually measured using a zero-calibration barometer transmitter.
Classic pressure gauges
Most pressure gauges belong to manometers or the class of elastic pressure sensors. Manometers use a liquid column to measure pressure. The manometer mechanism applies the measured pressure to the liquid column and the change in liquid level is measured. The use of a column of liquid limits the use of manometers to near-atmospheric pressure. Piezometer tubes, U-tube manometers, and slope tube barometers are examples of simple manometers. Elastic pressure sensors use an elastic element to measure pressure. The measured pressure puts pressure on an elastic element and the resulting deformation produces a signal proportional to the pressure. Most of the time, the primary measurement elements in local indicators or in electronic transmitters are elastic pressure sensors. Bourdon tubes, strain gauges, diaphragms and pressure gauges are elastic pressure sensors. The following sections present the operating principles of liquid manometers and Bourdon tube pressure gauges. These devices are classic pressure gauges and understanding how they work will help you understand the physics of pressure gauges. Discussion section of the former. 3.1 Fully describes the strain gauges, which are a type of elastic pressure sensor.
U-tube barometer
The U-tube barometer is one of the oldest and simplest pressure gauges. The main element of U-tube manometers is a U-shaped glass or plastic tube that contains a liquid such as water or mercury. The liquid is selected so that the process does not react when it comes in contact with the liquid (Figure 3-11). One end of the tube is open to the atmosphere and the process fluid puts pressure on the other end of the tube. This pressure compresses the manometer fluid and causes the tube to rise proportionally. The height or head, where the manometer fluid rises from the point of contact with the process fluid, is proportional to the pressure of the process fluid. Using Equation (3-8) you can convert the height of the liquid into a barometer.
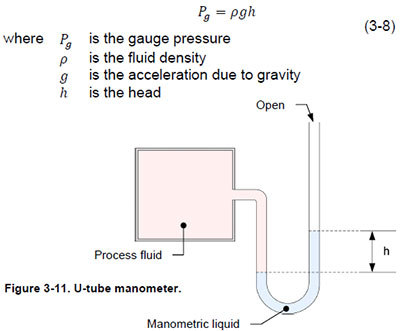
Manufacturers of U-tube barometers must carefully select the manometer fluid to ensure optimal measurement accuracy. The use of water manometers is limited to measuring pressure close to atmospheric pressure because a low pressure change causes a relatively large displacement of water. For example, to measure a 7 kPa (1 psig) barometer with a water barometer, the manometer column must be more than 71 cm (28 inches) high. The manufacturer can use mercury instead of water to significantly increase the measurement of a manometer. The density of mercury is 13.6 times the density of water. Thus, for a given pressure, the displacement of mercury is 13.6 times less than the displacement of water. Liquid pressure measurement is accurate enough to serve as a standard for calibrating other pressure gauges.
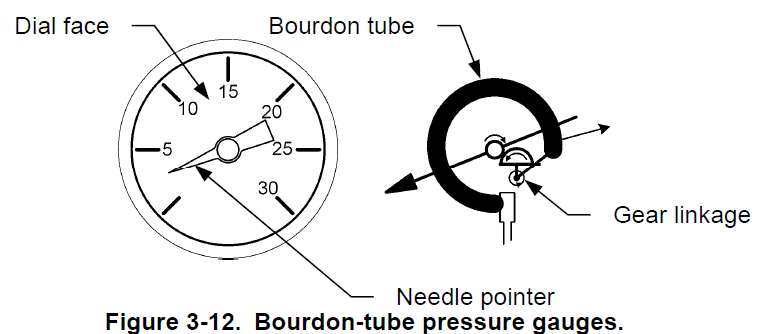
Bourdon pipe pressure
The Bourdon sphygmomanometer provides a direct pressure reading tube. They use an element of initial measurement called a Bourdon tube to sense pressure. Figure 3-12 shows a typical Bourdon tube barometer. The pressure gauge consists of a needle indicator connected to a Bourdon tube via a gear connection, which is a flexible C-shaped coil tube. The Bourdon tube is hollow and connects directly to the process fluid line. As the pressure increases, the Bourdon tube flattens, which moves the gear link and causes the needle pointer to dial. Bourdon pipe is made of materials that have elastic properties so that it deforms under pressure and returns to its original shape when it is no longer under pressure .